Tech
How Does Blood Hemolyzing Affect Bioanalysis Accuracy?
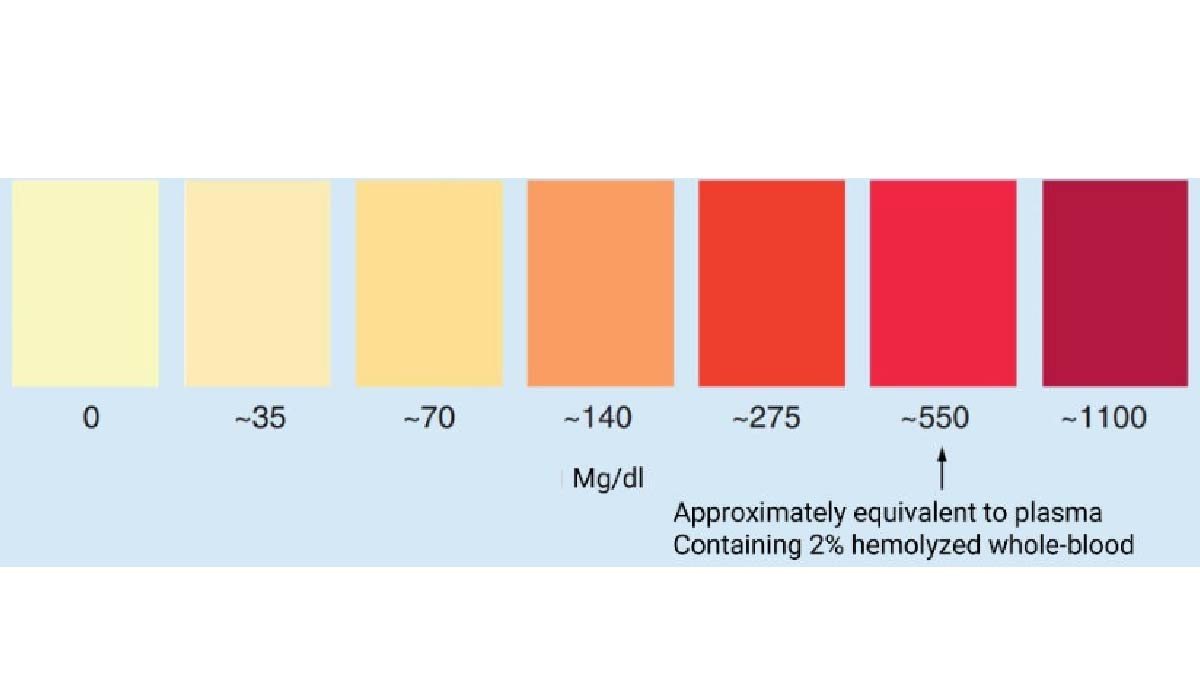
Blood hemolyzing disrupts sample integrity and creates challenges for accurate bioanalysis. When red blood cells rupture, they alter matrix composition, interfere with assays, and distort quantification. These changes impact PK studies, biomarker interpretation, and metabolic profiling. Understanding how hemolysis affects analytical methods helps laboratories apply safeguards that ensure reliable data, reduce variability, and support confident decision-making throughout drug development.
Biochemical Changes Caused by Hemolyzed Samples
Release of Intracellular Components Into the Matrix
Hemolysis releases potassium, enzymes, and other intracellular components into the sample matrix. These substances alter normal plasma or serum composition and interfere with measurements. Their presence can mask true analyte levels or create artificial spikes. Understanding these changes helps teams assess whether a sample reflects actual biological conditions or is compromised by cell rupture, allowing more accurate interpretation of study results.
Hemoglobin Interference With Analytical Assays
Hemoglobin strongly absorbs light and disrupts many spectrophotometric and chromatographic assays. Its presence changes optical density, affects peak resolution, and alters reaction kinetics. These interferences distort calibration curves and lead to unreliable quantification. Recognizing hemoglobin’s influence helps labs adjust assay conditions, apply correction factors, or exclude compromised samples to maintain accuracy in demanding bioanalytical workflows.
Shifts in Protein and Enzyme Levels After Hemolysis
Hemolysis causes major changes in protein and enzyme concentrations as cellular contents mix into the surrounding matrix. Enzymes such as LDH, AST, and ALT rise sharply, while protein distribution shifts due to membrane breakdown. These changes affect binding interactions, clearance estimates, and metabolite detection. Understanding these shifts helps analysts interpret unexpected results and avoid misclassification during PK or biomarker studies.
Assay Sensitivity and Selectivity Challenges
Matrix Effects That Distort Quantification
Hemolyzed samples introduce matrix effects that suppress or enhance signals during analysis. Interfering substances from ruptured cells compete with analytes during ionization or detection. This interaction leads to biased calibration and inaccurate quantification. Recognizing matrix-related distortions helps analysts adjust sample preparation, refine extraction methods, or redesign workflows to maintain selectivity and produce reliable, reproducible results.
Instrumental Signal Suppression or Enhancement
Mass spectrometry and other detection systems face signal suppression or enhancement when hemoglobin and cellular debris enter the sample stream. These materials influence ionization efficiency and fragment behavior, distorting measured intensities. Even small levels of hemolysis may cause significant shifts. Understanding instrument-level impacts helps teams apply dilution strategies, adjust tuning parameters, or remove compromised samples from analysis.
Reduced Reproducibility in Complex Bioanalytical Methods
Hemolysis introduces variability that reduces reproducibility in multi-step bioanalytical methods. Shifts in matrix composition affect extraction efficiency, chromatography performance, and detector response. These inconsistencies lead to higher variability across replicates and weaken confidence in study conclusions. By recognizing how hemolysis affects method consistency, analysts take steps to refine processes and ensure stable, repeatable results.
Impact on PK, Biomarker, and Drug Metabolism Studies
Variability in Drug Concentration Measurements
Drug concentration measurements depend on stable matrices. Hemolysis changes sample composition, influencing extraction recovery and detection. These shifts cause artificial increases or decreases in measured levels. In PK studies, such variability affects clearance estimates, half-life calculations, and exposure curves. Understanding hemolysis-related deviations helps teams exclude compromised samples and maintain accurate pharmacokinetic assessments.
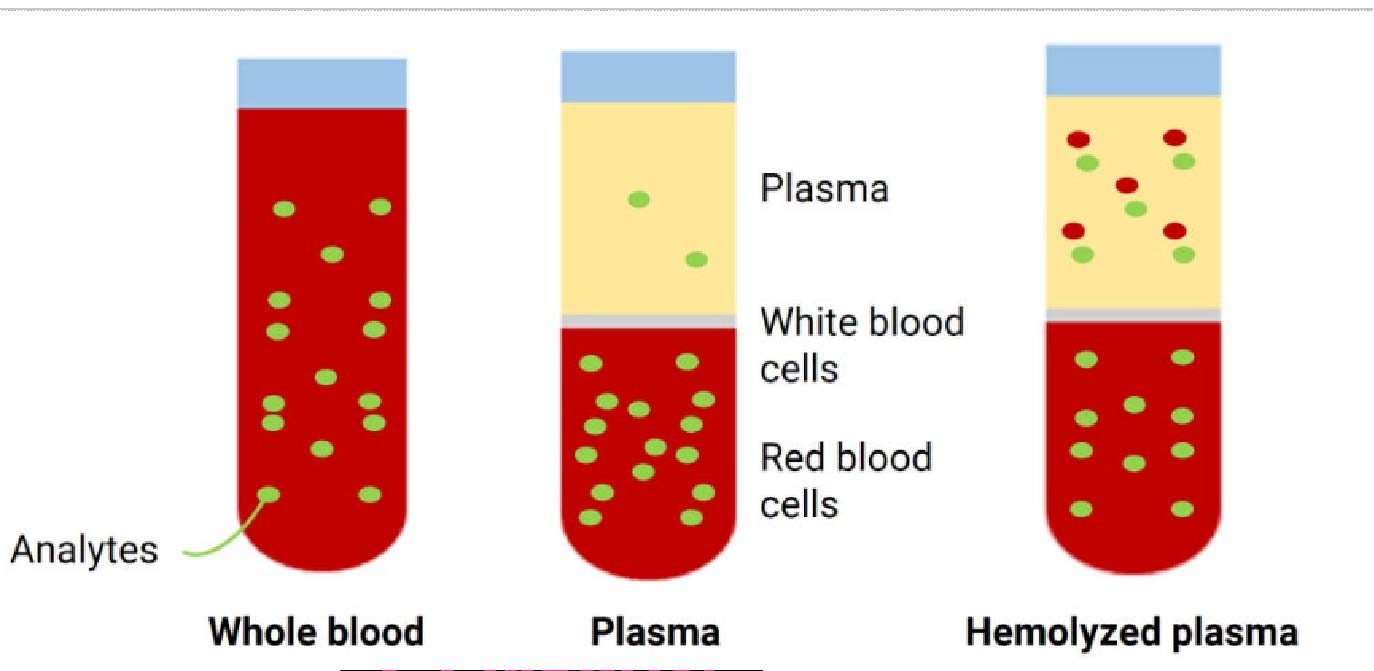
Misinterpretation of Biomarker Trends Due to Hemolysis
Biomarkers often respond to disease or treatment, but hemolysis may create false signals. Released enzymes and proteins distort biomarker concentrations and lead to misleading trends. These artifacts can suggest improvement, decline, or pathology unrelated to the study. Identifying hemolysis-induced changes helps researchers avoid misinterpretation and ensures biomarker data truly reflects physiological conditions.
Compromised Data Integrity in Metabolic Profiling
Metabolite studies require precise measurements of metabolic pathways and product distribution. Hemolysis introduces extra metabolites, enzymes, and hemoglobin fragments that complicate profiling. These interferences obscure metabolic signatures and contaminate chromatographic peaks. When analysts understand how hemolysis affects profiling, they refine sample preparation and apply rigorous controls to maintain data integrity throughout metabolism-focused research.
Best Practices to Reduce Hemolysis in Bioanalysis
Controlled Sample Collection and Immediate Handling
Proper collection minimizes mechanical stress and prevents premature cell rupture. Using the correct needle size, avoiding excessive suction, and gently mixing tubes protect sample integrity. Immediate handling and timely processing reduce exposure to harmful conditions. When teams follow controlled steps, they limit hemolysis risk and ensure samples remain suitable for accurate and consistent bioanalysis.
Quality Checks to Flag Hemolyzed Specimens Early
Visual inspection and automated hemolysis indices help analysts detect compromised samples before analysis begins. Early identification prevents wasted resources, avoids inaccurate reporting, and protects study integrity. Tracking hemolysis trends also reveals training gaps or equipment issues. Implementing routine checks strengthens quality systems and supports reliable bioanalytical performance across diverse study types.
Workflow Optimization to Maintain Sample Integrity
Optimizing workflows ensures samples remain stable from collection to analysis. Balanced centrifugation, temperature control, and gentle processing reduce stress on cells. Consistent protocols limit variation and help maintain reliable matrices for sensitive assays. When labs refine each step, they protect sample quality and improve the accuracy and reproducibility of bioanalysis outputs.
Conclusion
The blood hemolyzing disrupts matrix composition, interferes with assays, and introduces variability that complicates quantitative analysis. Understanding its biochemical and analytical impacts helps teams strengthen workflows and protect data quality. Early detection, controlled handling, and optimized methods reduce hemolysis-related errors. A proactive approach ensures bioanalysis results remain accurate, reproducible, and reliable across PK, biomarker, and metabolic studies.
-

 Celebrity1 year ago
Celebrity1 year agoWho Is Jennifer Rauchet?: All You Need To Know About Pete Hegseth’s Wife
-

 Celebrity1 year ago
Celebrity1 year agoWho Is Mindy Jennings?: All You Need To Know About Ken Jennings Wife
-

 Celebrity1 year ago
Celebrity1 year agoWho Is Enrica Cenzatti?: The Untold Story of Andrea Bocelli’s Ex-Wife
-

 Celebrity1 year ago
Celebrity1 year agoWho Is Klarissa Munz: The Untold Story of Freddie Highmore’s Wife